What Modification of Compound 1 Will Favor Transport of Na+ Relative to K+?
In cellular biology, membrane transport refers to the drove of mechanisms that regulate the passage of solutes such as ions and small molecules through biological membranes, which are lipid bilayers that incorporate proteins embedded in them. The regulation of passage through the membrane is due to selective membrane permeability - a characteristic of biological membranes which allows them to separate substances of distinct chemical nature. In other words, they tin can be permeable to sure substances only not to others.[1]
The movements of most solutes through the membrane are mediated by membrane transport proteins which are specialized to varying degrees in the transport of specific molecules. Equally the diversity and physiology of the distinct cells is highly related to their capacities to attract different external elements, it is postulated that there is a group of specific transport proteins for each cell type and for every specific physiological stage[ane]. This differential expression is regulated through the differential transcription of the genes coding for these proteins and its translation, for instance, through genetic-molecular mechanisms, but also at the cell biology level: the product of these proteins can exist activated by cellular signaling pathways, at the biochemical level, or even by being situated in cytoplasmic vesicles.[two]
Background [edit]
Thermodynamically the menstruation of substances from ane compartment to some other tin occur in the management of a concentration or electrochemical gradient or against it. If the exchange of substances occurs in the direction of the gradient, that is, in the direction of decreasing potential, there is no requirement for an input of free energy from outside the organization; if, all the same, the transport is against the gradient, it will require the input of energy, metabolic energy in this case.[iii] For instance, a classic chemical machinery for separation that does not crave the addition of external energy is dialysis. In this system a semipermeable membrane separates 2 solutions of different concentration of the same solute. If the membrane allows the passage of water but not the solute the water will move into the compartment with the greatest solute concentration in order to establish an equilibrium in which the energy of the system is at a minimum. This takes place considering the water moves from a loftier solvent concentration to a low one (in terms of the solute, the opposite occurs) and because the water is moving along a slope at that place is no need for an external input of free energy.

Diagram of a prison cell membrane
1. phospholipid 2. cholesterol 3. glycolipid 4. carbohydrate 5. polytopic protein (transmembrane protein) half-dozen. monotopic protein (here, a glycoprotein) 7. monotopic protein anchored by a phospholipid viii. peripheral monotopic protein (hither, a glycoprotein.)
The nature of biological membranes, especially that of its lipids, is amphiphilic, as they class bilayers that contain an internal hydrophobic layer and an external hydrophilic layer. This structure makes send possible by simple or passive diffusion, which consists of the improvidence of substances through the membrane without expending metabolic energy and without the aid of ship proteins. If the transported substance has a net electric charge, it will motility not only in response to a concentration gradient, but also to an electrochemical slope due to the membrane potential.
| Type of substance | Examples | Behaviour |
|---|---|---|
| Gases | CO2, N2, Oii | Permeable |
| Modest uncharged polar molecules | Urea, water, ethanol | Permeable, totally or partially |
| Big uncharged polar molecules | glucose, fructose | Non permeable |
| Ions | Thou+, Na+, Cl−, HCO3 − | Not permeable |
| Charged polar molecules | ATP, amino acids, glucose-half-dozen-phosphate | Non permeable |
As few molecules are able to lengthened through a lipid membrane the bulk of the transport processes involve transport proteins. These transmembrane proteins possess a large number of alpha helices immersed in the lipid matrix. In bacteria these proteins are nowadays in the beta lamina form.[4] This structure probably involves a conduit through hydrophilic poly peptide environments that crusade a disruption in the highly hydrophobic medium formed by the lipids.[1] These proteins can be involved in ship in a number of means: they human action as pumps driven by ATP, that is, by metabolic free energy, or as channels of facilitated diffusion.
Thermodynamics [edit]
A physiological process can only take place if it complies with basic thermodynamic principles. Membrane transport obeys concrete laws that define its capabilities and therefore its biological utility.
A general principle of thermodynamics that governs the transfer of substances through membranes and other surfaces is that the exchange of complimentary energy, ΔG, for the transport of a mole of a substance of concentration Cane in a compartment to another compartment where information technology is present at Cii is:[5]
When Cii is less than C1, ΔG is negative, and the process is thermodynamically favorable. As the energy is transferred from one compartment to some other, except where other factors intervene, an equilibrium will be reached where C2=C1, and where ΔYard = 0. Withal, there are three circumstances under which this equilibrium will not be reached, circumstances which are vital for the in vivo functioning of biological membranes:[5]
- The macromolecules on one side of the membrane tin can bond preferentially to a sure component of the membrane or chemically modify it. In this mode, although the concentration of the solute may actually be different on both sides of the membrane, the availability of the solute is reduced in one of the compartments to such an extent that, for practical purposes, no gradient exists to drive transport.
- A membrane electrical potential tin can exist which can influence ion distribution. For case, for the send of ions from the exterior to the interior, it is possible that:
Where F is Faraday's constant and ΔP the membrane potential in volts. If ΔP is negative and Z is positive, the contribution of the term ZFΔP to ΔG will be negative, that is, it volition favor the transport of cations from the interior of the cell. So, if the potential deviation is maintained, the equilibrium state ΔGrand = 0 will not stand for to an equimolar concentration of ions on both sides of the membrane.
- If a process with a negative ΔM is coupled to the transport process then the global ΔK volition be modified. This situation is common in active send and is described thus:
Where ΔGb corresponds to a favorable thermodynamic reaction, such as the hydrolysis of ATP, or the co-transport of a compound that is moved in the direction of its slope.
Transport types [edit]
Passive diffusion and active diffusion [edit]
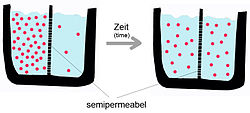
[half dozen]A semipermeable membrane separates two compartments of different solute concentrations: over time, the solute volition diffuse until equilibrium is reached.
As mentioned above, passive diffusion is a spontaneous phenomenon that increases the entropy of a system and decreases the gratuitous energy.[v] The transport procedure is influenced past the characteristics of the transport substance and the nature of the bilayer. The diffusion velocity of a pure phospholipid membrane will depend on:
- concentration slope,
- hydrophobicity,
- size,
- charge, if the molecule has a cyberspace charge.
- temperature
Active and co-transport [edit]
In active transport a solute is moved against a concentration or electrochemical gradient; in doing then the send proteins involved consume metabolic free energy, usually ATP. In primary active transport the hydrolysis of the energy provider (e.g. ATP) takes place directly in guild to transport the solute in question, for instance, when the ship proteins are ATPase enzymes. Where the hydrolysis of the energy provider is indirect as is the example in secondary active ship, employ is fabricated of the free energy stored in an electrochemical slope. For case, in co-transport use is made of the gradients of certain solutes to transport a target compound against its gradient, causing the dissipation of the solute gradient. It may announced that, in this example, there is no energy use, merely hydrolysis of the energy provider is required to constitute the gradient of the solute transported along with the target compound. The gradient of the co-transported solute will be generated through the use of certain types of proteins called biochemical pumps.[2]
The discovery of the existence of this type of transporter protein came from the study of the kinetics of cross-membrane molecule ship. For certain solutes it was noted that the transport velocity reached a plateau at a item concentration above which there was no pregnant increase in uptake rate, indicating a log curve type response. This was interpreted as showing that transport was mediated by the formation of a substrate-transporter complex, which is conceptually the same as the enzyme-substrate complex of enzyme kinetics. Therefore, each transport protein has an affinity constant for a solute that is equal to the concentration of the solute when the send velocity is one-half its maximum value. This is equivalent in the case of an enzyme to the Michaelis–Menten constant.[7] [viii]
Some important features of active transport in add-on to its ability to intervene even against a gradient, its kinetics and the use of ATP, are its high selectivity and ease of selective pharmacological inhibition[vii]
Secondary agile transporter proteins [edit]
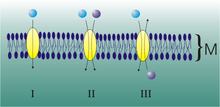
Uniport, symport, and antiport of molecules through membranes.
Secondary active transporter proteins motion two molecules at the same time: one confronting a gradient and the other with its slope. They are distinguished according to the directionality of the two molecules:
- antiporter (also called exchanger or counter-transporter): move a molecule confronting its gradient and at the same time displaces one or more ions along its gradient. The molecules move in opposite directions.
- symporter: motion a molecule confronting its gradient while displacing one or more different ions along their gradient. The molecules move in the same management.
Both can be referred to as co-transporters.
Pumps [edit]

A pump is a poly peptide that hydrolyses ATP to transport a particular solute through a membrane, and in doing so, generating an electrochemical gradient membrane potential. This gradient is of involvement every bit an indicator of the country of the prison cell through parameters such every bit the Nernst potential. In terms of membrane transport the gradient is of interest as it contributes to decreased system entropy in the co-ship of substances against their gradient. 1 of the near of import pumps in animal cells is the sodium potassium pump, that operates through the following mechanism:[9]
- binding of three Na+ ions to their agile sites on the pump which are jump to ATP.
- ATP is hydrolyzed leading to phosphorylation of the cytoplasmic side of the pump, this induces a structure alter in the protein. The phosphorylation is acquired by the transfer of the terminal group of ATP to a residual of aspartate in the transport protein and the subsequent release of ADP.
- the structure modify in the pump exposes the Na+ to the outside. The phosphorylated grade of the pump has a depression affinity for Na+ ions then they are released.
- once the Na+ ions are liberated, the pump binds 2 molecules of K+ to their respective bonding sites on the extracellular face up of the transport poly peptide. This causes the dephosphorylation of the pump, reverting information technology to its previous conformational state, transporting the M+ ions into the jail cell.
- The unphosphorylated form of the pump has a higher affinity for Na+ ions than K+ ions, then the two bound K+ ions are released into the cytosol. ATP binds, and the process starts again.
Membrane selectivity [edit]
As the main feature of transport through a biological membrane is its selectivity and its subsequent beliefs as a barrier for certain substances, the underlying physiology of the miracle has been studied extensively. Investigation into membrane selectivity have classically been divided into those relating to electrolytes and non-electrolytes.
Electrolyte selectivity [edit]
The ionic channels define an internal diameter that permits the passage of modest ions that is related to diverse characteristics of the ions that could potentially exist transported. As the size of the ion is related to its chemic species, it could be assumed a priori that a aqueduct whose pore diameter was sufficient to allow the passage of one ion would as well permit the transfer of others of smaller size, nonetheless, this does not occur in the majority of cases. In that location are 2 characteristics alongside size that are of import in the determination of the selectivity of the membrane pores: the facility for dehydration and the interaction of the ion with the internal charges of the pore.[7]
In society for an ion to pass through a pore information technology must dissociate itself from the water molecules that cover information technology in successive layers of solvation. The trend to dehydrate, or the facility to do this, is related to the size of the ion: larger ions can practice it more than hands that the smaller ions, so that a pore with weak polar centres will preferentially allow passage of larger ions over the smaller ones.[7] When the interior of the aqueduct is equanimous of polar groups from the side chains of the component amino acids,[9] the interaction of a dehydrated ion with these centres can be more important than the facility for dehydration in conferring the specificity of the channel. For example, a channel fabricated upwards of histidines and arginines, with positively charged groups, will selectively repel ions of the same polarity, but will facilitate the passage of negatively charged ions. Also, in this instance, the smallest ions volition exist able to interact more than closely due to the spatial arrangement of the molecule (stericity), which profoundly increases the charge-accuse interactions and therefore exaggerates the effect.[7]
Non-electrolyte selectivity [edit]
Not-electrolytes, substances that mostly are hydrophobic and lipophilic, usually laissez passer through the membrane by dissolution in the lipid bilayer, and therefore, by passive diffusion. For those not-electrolytes whose transport through the membrane is mediated by a transport protein the ability to diffuse is, generally, dependent on the partition coefficient Thousand. Partially charged non-electrolytes, that are more or less polar, such as ethanol, methanol or urea, are able to pass through the membrane through aqueous channels immersed in the membrane. In that location is no effective regulation mechanism that limits this send, which indicates an intrinsic vulnerability of the cells to the penetration of these molecules.[7]
Creation of membrane send proteins [edit]
There are several databases which attempt to construct phylogenetic trees detailing the creation of transporter proteins. Ane such resource is the Transporter Classification database[10]
Meet also [edit]
- Cellular transport
References [edit]
- ^ a b Lodish; et al. (2005). Biología celular y molecular (Buenos Aires: Médica Panamericana ed.). ISBN950-06-1374-3.
- ^ a b Alberts; et al. (2004). Biología molecular de la célula (Barcelona: Omega ed.). ISBN84-282-1351-8.
- ^ Cromer, A.H. (1996). Física para ciencias de la vida (in Castilian) (Reverté ediciones ed.). ISBN84-291-1808-X.
- ^ Prescott, Fifty.M. (1999). Microbiología (McGraw-Hill Interamericana de España, South.A.U. ed.). ISBN84-486-0261-7.
- ^ a b c Mathews C. K.; Van Holde, K.E; Ahern, K.G (2003). Bioquímica (3rd ed.). ISBN84-7829-053-2.
- ^ Zaheri, Shadi and Hassanipour, Fatemeh. "A comprehensive arroyo to the mathematical modeling of mass send in biological systems: Fundamental concepts and models". International Periodical of Rut and Mass Transfer. 158: 119777.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d due east f Randall D; Burggren, W.; French, K. (1998). Eckert Fisiología creature (fourth ed.). ISBN84-486-0200-v.
- ^ "A comprehensive arroyo to the mathematical modeling of mass transport in biological systems: Fundamental concepts and models". International Journal of Estrus and Mass Transfer. 158: 199777.
- ^ a b Lehninger, Albert (1993). Principles of Biochemistry, 2nd Ed (Worth Publishers ed.). ISBN0-87901-711-2.
- ^ "Transporter Classification Database". Archived from the original on three January 2014. Retrieved 15 July 2010.
Source: https://en.wikipedia.org/wiki/Membrane_transport



0 Response to "What Modification of Compound 1 Will Favor Transport of Na+ Relative to K+?"
Post a Comment